If you are a student of 7th standered, the artical Acid Base and Salt is best for you. Here we describe the topic in a easy way
Acid: A substance with particular chemical properties including turning litmus red, neutralizing alkalis, and dissolving some metals are called acid.
Base: . A base is a substance that neutralizes acids. When bases are added to water, they split to form hydroxide ions.
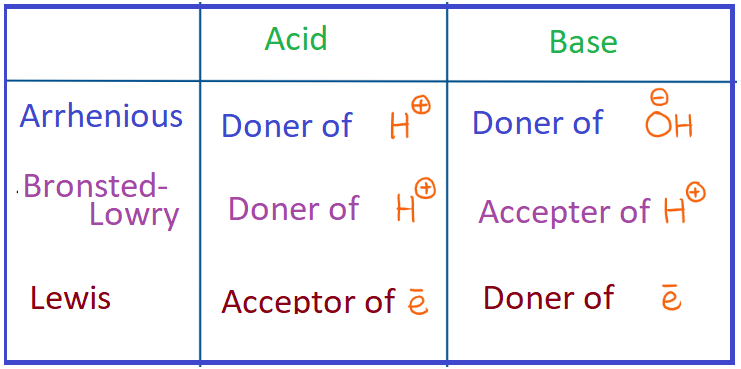
According to Arrhenius theory an __
Acid is a substance which has a hydrogen atom and which release hydrogen ion (H+) ions when dissolved in water. Such substances are called as Arrhenius acids .e.g. HCl.
Base is a substance which has a hydrogen atom and which release Hydroxyl ion (OH- ) ions when dissolved in water. Such substances are called as Arrhenius base .e.g NaOH.
According to Bronsted-Lowry theory,
acid is a substance which donates an H+ ion or a proton and forms its conjugate base and the base is a substance which accepts an H+ ion or a proton and forms its conjugate acid.
The properties of acid are as follows:
Acids are sour in taste.
Acids furnish hydrogen ions in aqueous solution.
Acid reacts with metal to form hydrogen gas.
Acid reacts with carbonates and liberates carbon dioxide gas.
Blue litmus turns red in acid
Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current
The Properties of Bases
Bases change the colour of litmus from red to blue.
They are bitter in taste.
Bases lose their basicity when mixed with acids.
Bases react with acids to form salt and water. …
They can conduct electricity.
Bases feel slippery or soapy.
Some bases are great conductors of electricity
Indicator:
Indicators are substances that are used to test whether a substance is acidic or basic ot neutral in nature. They change their color when added to a solution containing an acidic or a basic substance. Indicators are of two types – natural and artificial. Naturally occurring indicators are turmeric, litmus, china rose and red cabbage.
Litmus: Litmus is a natural dye extracted from lichens. It is the most commonly used natural indicator. Litmus turns acidic solutions red and basic solutions blue. Neutral solutions do not change the color of either red or blue litmus.
Turmeric: Turmeric gives brownish red color in basic medium and yellow in acidic medium.
China Rose: A solution of china rose turns green in a basic solution, and bright pink or magenta in an acidic solution.
Types of acid:
1. Based on the source of origin, the acid is classified as
i) Organic acid ii) Mineral acid or Inorganic acid.
Organic acid:
Organic acids are acids that are derived from plants and animals.
Example: Citric acid, Latic acid, Malic acid, Tannic acid
Mineral acid:
Mineral acids are acids that are derived from an inorganic substance or source.
Example: Nitric acid, Hydrochloric acid, Sulphuric acid
2. Depending on the quantity of water present in acids, it is classified as
i) Concentrated acids ii) Diluted acids
Concentrated acids: The concentration of acid is more compared to that of water. These are pure acids.
Diluted acids: Dilute acids are acids that contain high quantity of water than concentrated acids. They’re made by diluting a concentrated acid with waterr
Types of Bases:
Bases are graded as strong or weak based on how they participate in a reaction.
1. Strong bases: Some of the bases are corrosive and can cause skin irritation; they’re called strong bases—for example, potassium hydroxide, sodium hydroxide, and calcium hydroxide.
Note: The complete dissociation of OH− ions takes place in strong acids.
2. Weak bases: Some of the bases aren’t especially corrosive; they’re called weak bases—for example, magnesium hydroxide, ammonium hydroxide, copper hydroxide. The partial dissociation of OH− ions takes place.
pH:
pH is a measure of how acidic/basic water is. The abbreviation for pH refers to the potential of hydrogen or the power of hydrogen. The range goes from 0 – 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water
Salt:
salt, in chemistry, substance produced by the reaction of an acid with a base. A salt consists of the positive ion (cation) of a base and the negative ion (anion) of an acid. The reaction between an acid and a base is called a neutralization reaction.
Conclusion: Based on the information we have collected and investigated, we have concluded that acids, bases and salts are very important in our daily lives as well as it also important to understand the science.
